Document Type : Original Article
Authors
1 Department of Aquatic Animal Health and Diseases, Caspian Sea Ecology Research Center, Sari, Iran
2 DVM Graduate, Faculty of Veterinary Medicine, Shahrekord University, Shahrekord, Iran
3 Department of Pathobiology, Faculty of Veterinary Medicine, Shahrekord University, Shahrekord, Iran
Abstract
Parasitic diseases are harmful and limiting factors in breeding and rearing ornamental fish industry. In this study, 400 apparently healthy ornamental fishes from five species (each species 80 specimens) including: Goldfish (Carassius auratus), guppy (Poecilia reticulate), angelfish (Pterophyllum scalare), discus (Symphsodon discus) and sailfin mollies (Poecilia latipinna) was obtained from a local ornamental fish farm in the north of Iran during 2011 to 2012. The primary purpose of this study was to determine the parasitic infections of aquarium fish in Iran. For this purpose, fish were first examined for ectoparasites using wet mount under a light microscope. Then, the alimentary ducts of fish were observed under light and stereo microscope. In survey of different infection rates for different parasitic infections in examining fish: Dactylogyrus sp., Gyrodactylus sp., Ichthyophthirius multifiliis Trichodina reticulata, Capillaria sp. and Lernaea cyprinacea were collected from five species. All five fish species had Monogenea (Gyrodactylidae and Dactylogyridae) in their skins and gills, the highest prevalence was observed in C. auratus and the lowest was in P. scalare and S. discus. Also, Capillaria sp. was reported as a first record from the abdominal cavity of P. scalare in Iran. Our findings revealed that the protozoal infections are very common among aquarium fishes. Although, no gross pathology was observed among infected fishes, but it is likely that in case of any changes in the environment, then parasitic infections could be harmful.
Keywords
Introduction
Aquarium fish trade is a very important sector all over the world.1 The global trade in ornamental fish, associated aquarium and pond accessories is more than 7 × 109 USD each year. They are a significant source of overseas benefit for many rustic communities in Africa, south America and south-east Asia.1 Thousands types of aquarium fish (commonly, poeciliids, guppy and cichlids) are collected and maintained by hobbyists.1 The biggest portion of the aquarium fish industry is the freshwater aquarium fish sector. Cultivation and propagation of ornamental fishes have been increased in the recent decades in Iran, for its beautiful appearance, the small size andeasy maintenance.1
Although this worldwide interest in ornamental fish has led to development in their cultivation techniques, there are still many difficult-to-culture species with high demand. Ornamental fish pathogens spread very rapidly in the world because of their commercial benefits. Consequently, routine infectious disease controls are very important for risk analysis and precaution steps. Parasites are harmful and limiting factors in breeding and rearing ornamental fish industry.2 From economic aspects, parasitic diseases in fish have a particular importance, because of causing sterility, discoloration, change of body shape and decreased growth and weight of fish.3 Therefore, knowledge about fish parasites is crucial for successful aquaculture. For this reason, we aimed to isolate and identify the parasitic fauna of five species of ornamental freshwater fish in northern Iran.
Materials and Methods
A total number of 400 apparently healthy ornamental fishes including Goldfish (Carassius auratus; n = 80), guppy (Poecilia reticulate; n = 80), angelfish (Pterophyllum scalare; n = 80), discus (Symphsodon discus; n = 80) and sailfin mollies (Poecilia latipinna; n = 80)were obtained from local ornamental fish farms in Mazandaran province (North of Iran) between 2011-2012, (Table 1). Live fishes were transferred to fish diseases laboratory at the Caspian Sea Ecology Research Center using portable air pump.
The external surface, abdominal cavities and digestive tracts were examined for presence of parasitic fauna. Fish were first examined for ectoparasites using wet mount under a light microscope (Olympus, Tokyo, Japan).1 Then, the alimentary ducts of fish were observed under light and stereo microscope. Parasites of alimentary tracts were counted and fixed in 70% ethanol, and for examination, they were cleared using glycerine.2 Identification of the parasites was carried out using the identification keys.2,4,5
Table 1. The geographical distribution of sampling in each examined fish species in the Mazandaran province, Iran.
|
Fish species |
Region/Location of sampling |
|
Pterophyllum scalare |
Sari, Tonekabon |
|
Carassius auratus |
Babolsar, Amol, Sari |
|
Symphsodon discus |
Feridonkenari, Tonekabon |
|
Poecilia latipinna |
Joibar, Sari, Babol |
|
Poecilia reticulata |
Tonekabon, Sari, Babolsar |
Results
During the sampling, the water temperature was 25 ± 3 ˚C, dissolved oxygen was 4.60 ± 0.50 mg L-1 and pH was 7.20 ± 0.60, respectively. Of all examined fishes, 380 fishes (95.00%) were infected by at least one parasite. One nematode (Capillaria sp.), two protozoa (I. multifilis and, Trichodina reticulata), two monogeneans (Dactylogyrus sp. and Gyrodactylus sp.) and one Crustacea (L. cyprinacea) were identified (Figs. 1 to 5 and Table 2). The hemorrhagic areas on the skin and gills, fins bleeding, scales losing and fin rot was observed in infected fish.

Fig. 1.Trichodina reticulata isolated from discus (400×).
Fig. 2. Two pairs of anchor hooks (arrow) of Gyrodactylus sp. isolated from guppy(100 ×).

Fig. 3. Anchors of Dactylogyrus sp. (Total length of anchor = 48.5 μm) isolated from Goldfish(100 ×).
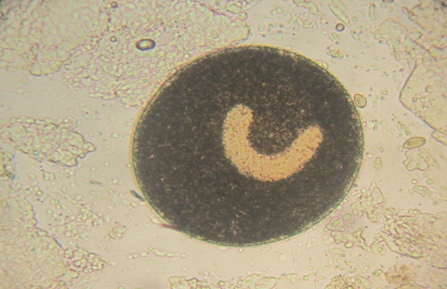
Fig. 4. Large horseshoe-shaped macronucleus of Ichthyophthirius multifiliis isolated from discus (100 ×).
Fig. 5. A female of Capillaria sp. with barrel-shaped eggs (arrow) in intestine of angelfish(100×).
Table 2. Parasitic fauna in ornamental fish in Mazandaran province according to this study.
|
Host |
Parasites |
Infected organ |
Infected fish (%) |
Range of infestation/Infection |
|
Pterophyllum scalare
|
Dactylogyrus sp. Gyrodactylus sp. Ichthyophthirius multifiliis Trichodina reticulata Capillaria sp. |
Gills Skin Skin Skin/Fin Intestine |
35.00 5.00 15.00 25.00 22.50 |
1-4 1-4 1-11 1-6 1-3 |
|
Carassius auratus |
Dactylogyrus sp. Gyrodactylus sp. Ichthyophthirius.multifiliis Trichodina reticulate Lernaea cyprinacea |
Gills Skin Skin Skin Skin/Fin |
28.75 72.50 87.50 20.00 30.00 |
1-4 1-8 1-16 1-14 1-3 |
|
Symphsodon discus |
Dactylogyrus sp. Gyrodactylus sp. Ichthyophthirius multifiliis Trichodina reticulate |
Gills Skin Skin/Fin Skin/Fin |
7.50 13.75 10.00 6.25 |
1-2 1-2 1-8 1-4 |
|
Poecilia latipinna |
Dactylogyrus sp. Gyrodactylus sp. Ichthyophthirius multifiliis Trichodina reticulata Capillaria sp. Lernaea cyprinacea |
Gills Skin Skin/Fin Skin/Fin Intestine Skin/Fin |
16.25 28.75 12.50 12.50 1.25 5.00 |
1-2 1-5 1-4 1-7 1-2 1 |
|
Poecilia reticulata
|
Dactylogyrus sp. Gyrodactylus sp. Ichthyophthirius multifiliis Trichodina reticulata Capillaria sp. Lernaea cyprinacea |
Gills Skin Skin/Fin Skin Intestine Skin/Fin |
17.5 21.25 6.25 15.00 2.50 1.25 |
1-3 1-2 1-6 1-9 1 1-2 |
Discussion
During the previous decades, fish parasites identification have become increasingly visible , because of the growth of freshwater ornamental fish industries throughout the world.2 Parasitic diseases affect physiologic and biologic characteristics, caused mechanical damage and economic losses in ornamental fish industries.2
Different parasite species were reported from various ornamental fish species around the world. Tetrahymena sp. was collected from gills of Carnegiella strigata, Piscinoodinium pilullare from the skin of Carnegiella martae, Trichodinids spp. from the skin of C. strigata,also Nannostomus and Procamallanus sp. was isolated from the intestine of Paracheirodon axelrodi.8Koyun reported Gyrodactylus katharine and Gyrodactylus carassii from the gills of C.carassius,9 Ichthyobodosp., I.multifiliis, Chilodonella sp., Trichodina spp from the skin., Dactylogyrus extensus, Gyrodactylus bullatarudis, L. cyprinacea, Argulus foliaceus, Argulus japonicus and Capillaria sp. from the external parts of goldfish, guppy and cichlids.10 Ambiphyra spp. was reported from the skin of guppy,11 and also, Oodinium pillularis was isolatedfrom the skin of Poecilidae.12
In Iran, there were also many reports of parasite fauna from ornamental fishes for example, Meshgi et al. reported Dactylogyrus rotator, Chiloldonella sp., Hexamita sp., Ictyobodonecator, I.multifiliis, Microsporidium,Myxosporidasp., Tricodina spp., and L. cyprinicea from Aquarium fishes around Tehran.1
Ichthyophthirius multifiliis, Gyrodactylus sp., Dactylogyrus sp., Trichodina spp., Argulus coregoni, A. japonicas, A. foliaceus was reported from C. auratus.13 Also, I. multifiliis, Dactylogyrus sp., Microsporidian sp. and Ichthyobodo sp. were reported from angelfish in the Mazandaran province.14
In this study, I. multifiliis had the highest infection rate in C. auratus. The highest prevalence of Gyrodactylidae and Dactylogyridae were observed in C. auratus and the lowest in P. scalare and S. discus, respectively.
In our study, Capillaria sp. was reported for the first time from P. scalare in Iran. This nematode may cause high mortality in aquarium fishes. Rahmati-holasoo et al. showed that infection with Capillaria sp. could cause a great loss in ornamental fish from Cichlidae in Iran. 15
It seems that many factors such as water quality, fish density, diet, physiology of host and parasite life cycle may have contributed to the severity and type of these parasites.2 Given the important role of risk factors, reducing stressful situations through improved management and environmental conditions such as improved water quality and switch on time, reduction of organic matter, avoiding excessive density of fish and unnecessary manipulation and using appropriate disinfectants in farms can be useful to control and reduce economic losses caused by parasitic disease in ornamental fishes.
The identified parasites in this study have not been reported as a parasitic problem in Iran. However, the rate of infection in these aquarium fishes was low. The possibility of transmition of contamination to the native aquarium fishes, even farmed fishes should be taken into consideration.
Acknowledgements
The authors appreciate the Department of Clinical Sciences, Aquatic Animal Health and Diseases, Research Organization of Caspian Sea, Sari, Iran for their assistance.
- Meshgi B, Eslami A, Yazdani H. Study on the parasitic infections of aquarium fishes around Tehran. J Vet Res 2006; 61(1): 1-5.
- Jalali B. Parasites and parasitic diseases of fresh water fishes of Iran. Iranian fisheries research organization. Tehran, Iran: 1997; 105-112.
- Moravec F. Parasitic nematodes of fresh water fishes of Europe. Prague, Czech Republic: Academia Press 1994; 473.
- Bauer ON. Key to the parasites of the fresh water fish fauna of the USSR [In Russian]. Leningrad, Russia: Nauka 1987; 300-312.
- Moravec F, Orecchia P, Paggi L. Pseudcapillaria parablennii sp. (Nematoda: Capillariidae) from a marine fish, Parablennius gattorugine (Brunn), from the Italian Coast. Folia Parasitol 1988; 35: 353-357.
- Winfree R. Tropical fish: Their production and marketing in the United States. World Aquacult 1989; 20:24-30.
- Noga EJ. Fish disease, diagnosis and treatment. 2th ed. Ames, USA: Wiley Blackwell Publishing 2010; 536.
- Tavares-Dias M, Gonzaga Lemos R, Laterça J, et al. Parasitic fauna of eight species of ornamental freshwater fish species from the middle Negro river in the Brazilian Amazon region. Rev Bras Parasitol 2010; 19(2): 103-107.
- Koyun M. The helminthes fauna of some fishes in Enne Dam Lake. Phd Thesis, Uludag University Science Institution. Bursa, Turkey: 2000.
- Koyuncu CE. Parasites of ornamental fish in Turkey. Bull Euro Assoc Fish Pathol 2009; 29: 25-27.
- Kayis S, Ozcelep T, Capkin E, et al. Protozoan and metazoan parasites of fish in the Turkey and their applied treatment. Israel J Aquatic Bamid 2009; 61: 93-102.
- Koyuncu E, Cengizler I. Common protozoan ecto-parasites in some growing aquarium fish (Poecilidae) in Mersin region: A case study [Turkish]. J Fish Aquat Sci 2002; 19: 293-301.
- Ebrahimzadeh Mousai HA, Behtasg F, Rostami M, et al. Study of Arguus spp. Infestation rate in goldfish, Carassius auratus (Linnaeus, 1758) in Iran. HVM Bioflux Society 2011; 3(3): 198-204.
- Taherpour SM, Brimani M, Nejati Saravi A, et al. Survey Infection parasites of freshwater ornamental fishes in Sari province. In proceedings: The 17th Iranian veterinary congress. Tehran, Iran. 2012; 222.
- Rahmati-holasoo H, Ebrahimzadeh Mousavi H, Soltani M, et al. Capillariosis in Breeder Discus (Symphysodon aequifasciatus) in Iran. J Agri Sci 2010; 5(3): 253-259.

